PMG
About us.
PMG provides a full suite of services, including precision glass components, ceramics and sapphire, hermetic sealing, optical coatings, assembly and bonding. We provide the expertise and resources needed to ensure your innovation achieves its full potential.
What we do.
Precision Medical Glass (PMG) is an ISO 13485 certified company dedicated to providing optical, non-optical and technical ceramic components to medical OEMS, contract manufacturers and research labs across the globe.
PMG was spun out of IRD Glass in 2022 to provide the same level of service but with a focus on the medical markets desiring the ISO 13485 certification. With the legacy of IRD Glass to lean on, PMG is set to provide unparalleled quality, value, and service.
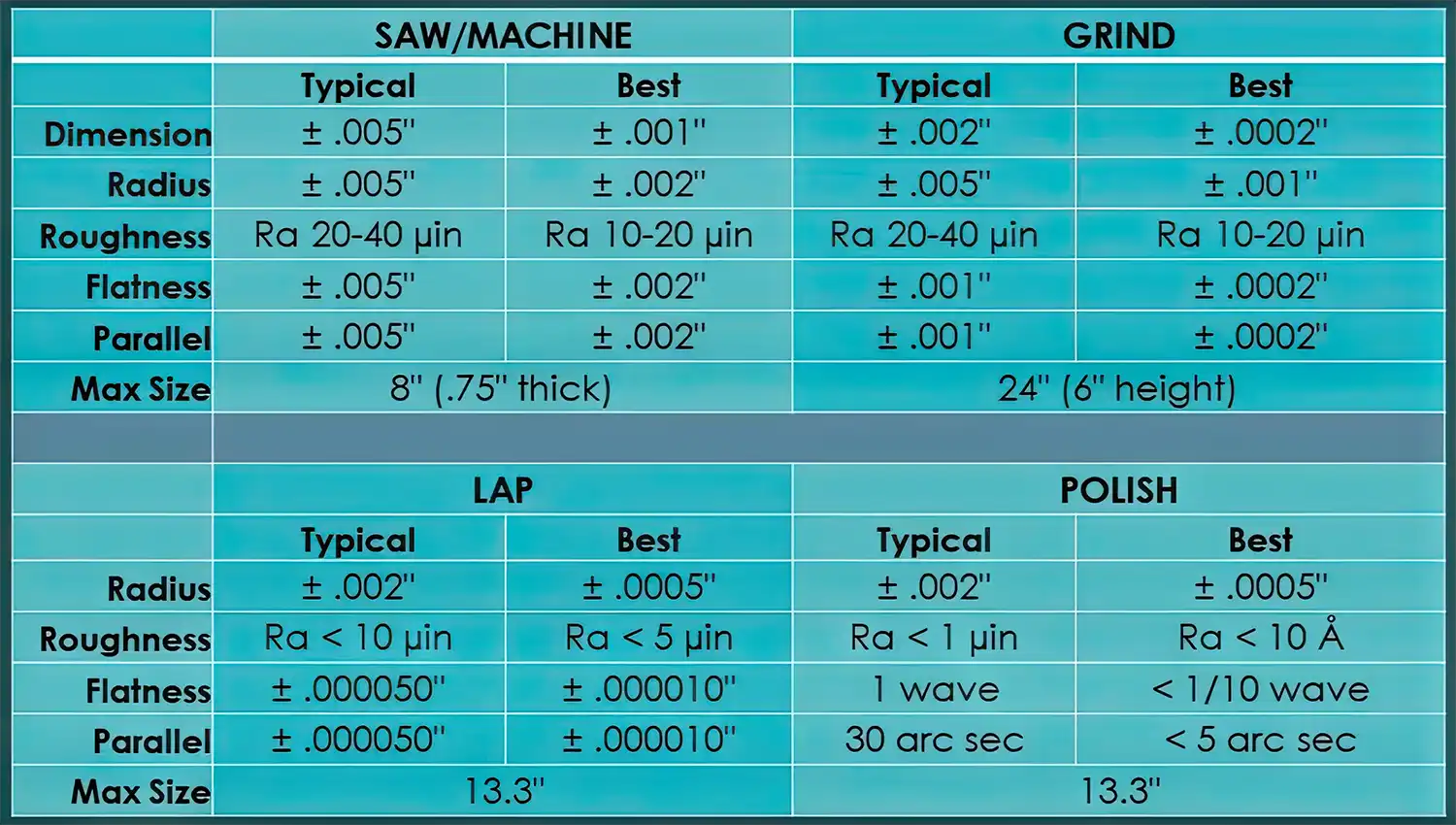
Capabilities.
Precision Medical Glass's (PMG) core capabilities encompass manufacturing precision components from glass, sapphire, and technical ceramic materials. Processes include sawing, dicing, grinding, machining, drilling, lapping, and polishing. PMG is not certified to design products but excels at taking your vision and making it a reality. PMG excels at design for manufacturability. Taking your initial design and making recommendations that may offer additional value is a strength we gladly share with our partners.
Materials.
PMG has experience working with a vast portfolio of glass types in the visible and IR spectrum. PMG is also experienced with most types of technical ceramics; machining, and polishing to exacting tolerances.

Optical Materials.
Fused Silica - Fused Quartz - nBK7 and equivalents - SF glass - Schott filter glass - Hoya Filter glass - Isuzu filter glass - Kopp filter glass - Borofloat - B270 - D263 - Eagle - Gorilla - Dragontail - Zerodur - ULE - Silicon -Chalcogenide - Sapphire - Simax - Spinel - Alon
Technical Ceramics.
Macor - Alumina - Zirconia - Sapphire - Aluminum Nitrite - Silicon Nitride - Silicon Carbide - Spinel - Alon
Values.
Our core values are the biblically-based bedrock that our decisions and Actions are built upon. These values are our legacy and our future. We embrace and live these values such that they are apparent to any person who interacts with us.
Pride
We show integrity in everything we do and take responsibility to reach the highest quality level.
Innovation
We share a passion for continuous improvement and never-ending innovation in all aspects of our lives.
Partnership
We demonstrate goodwill, teamwork and selflessness making the work we do fulfilling and enjoyable.
Testimonials
Add your customer testimonial here. This is a rich text field so you can add links and format the text however you like.
Minneapolis
CTO, Technostuff
Add your customer testimonial here. This is a rich text field so you can add links and format the text however you like.
Colorado
Director, NFR Engineering
Add your customer testimonial here. This is a rich text field so you can add links and format the text however you like.
San Francisco
Innovation VP, Spike Technology
Certifications.
ISO 13485 vs ISO 9001
ISO 13485 and ISO 9001 are both important quality management system (QMS) standards, but they serve different purposes and are tailored to different industries. Here are the advantages of having an ISO 13485 certification compared to ISO 9001, particularly for companies in the medical device industry:
1. Industry-Specific Requirements
- ISO 13485: Explicitly tailored for the medical device industry, addressing the unique requirements for medical device manufacturing, including regulatory compliance, risk management, and product traceability.
- ISO 9001: A more general QMS standard applicable to any industry, focusing on customer satisfaction and continuous improvement without specific guidance on medical device regulations.
2. Regulatory Compliance
- ISO 13485: Aligns closely with global regulatory requirements for medical devices, including FDA regulations (21 CFR 820) in the United States, EU MDR in Europe, and other international medical device regulations. Certification can streamline the approval process in different markets.
- ISO 9001: While it demonstrates a commitment to quality management, it does not specifically address regulatory requirements for medical devices, which could require additional steps for compliance.
3. Risk Management
- ISO 13485 emphasizes a risk-based approach throughout a medical device’s lifecycle, including design, development, production, and post-market activities. This focus helps ensure its safety and efficacy.
- ISO 9001: Encourages a risk-based approach to quality management but does not specifically address the unique risks associated with medical devices.
4. Product Traceability
- ISO 13485: Requires comprehensive traceability for medical devices, from raw materials to final products, to ensure that any issues can be quickly identified and addressed. This is critical in the event of a recall or quality issue.
- ISO 9001: Includes general requirements for traceability but does not specify the level of detail needed for medical devices, which might be insufficient for regulatory purposes.
5. Focus on Medical Device Safety
- ISO 13485 strongly emphasizes the safety of medical devices, ensuring that the products meet stringent safety and performance standards required in healthcare.
- ISO 9001: This standard focuses on general quality management principles, such as customer satisfaction and continuous improvement, without specific attention to product safety in the context of medical devices.
6. Supplier Management
- ISO 13485: Requires stringent control of suppliers and subcontractors to ensure that all components and services meet medical device regulations, including detailed records of supplier evaluations and performance.
- ISO 9001: Also requires supplier management without the specific emphasis on medical device compliance, which may not be sufficient for highly regulated environments.
7. Document and Record Control
- ISO 13485: Includes specific requirements for document and record control, particularly regarding medical device files, technical documentation, and regulatory records, which are crucial for audits and inspections.
- ISO 9001: While it also mandates document and record control, it does not focus on the detailed documentation needs of the medical device industry.
8. Market Access
- ISO 13485: Often a prerequisite for selling medical devices in many international markets. Many regulatory bodies worldwide recognize ISO 13485 certification as part of their approval process.
- ISO 9001: Generally recognized as a good standard for quality management, but it may not be sufficient on its own to meet the regulatory requirements of the medical device industry.
9. Audit Readiness
- ISO 13485: Prepares companies for regulatory audits by ensuring that all processes, documentation, and quality controls meet the specific requirements of medical device regulations.
- ISO 9001: Prepares companies for general quality audits but may not fully prepare them for the specific demands of regulatory audits in the medical device sector.
10. Customer Confidence
- ISO 13485: Provides additional assurance to customers and partners that the company meets the highest standards specific to the medical device industry, which can be a significant competitive advantage.
- ISO 9001: Demonstrates a commitment to quality management but may not carry the same level of assurance in the medical device industry.
In summary, while both ISO 13485 and ISO 9001 are valuable, ISO 13485 offers specific advantages for companies involved in the design, development, and manufacturing of medical devices, particularly regarding regulatory compliance, risk management, and product safety.

Geneology.
In early 2019, a global medical prosthetic company was searching for a way to hermetically seal a passive sensor in a material that wouldn't interfere with MRI imaging. They contacted IRD Glass, a precision glass component manufacturer with over 40 years of experience building the impossible.
After working through the initial designs and honing the manufacturability aspects, IRD Glass decided to spin out a new company solely to focus on precision glass components for the medical field. This new entity would have an ISO 13485 certification to meet the medical field's stringent quality and traceability demands. And PMG was born.
PMG has decades of experience in IRD Glass to lean on for the manufacturing competencies and the additional capabilities that ISO 13485 demands. It is a perfect blend of skill and quality.
Meet our amazing team

Shanna Borowicz
Title

John Rutledge
Title

Dirk Kvale
Title

Lance Grobe
Title

Kurt Elfering
Title

Rebecca Nelson
Title

Aleysha Bartz
Title

Gerard Crosby
Title

Todd Anderson
Title

Troy Marco
Title

Riley Anderson
Title

Brandi Nelson
Title